YOUR BROWSER IS OUT-OF-DATE.
We have detected that you are using an outdated browser. Our service may not work properly for you. We recommend upgrading or switching to another browser.
PI Marcin Poreba
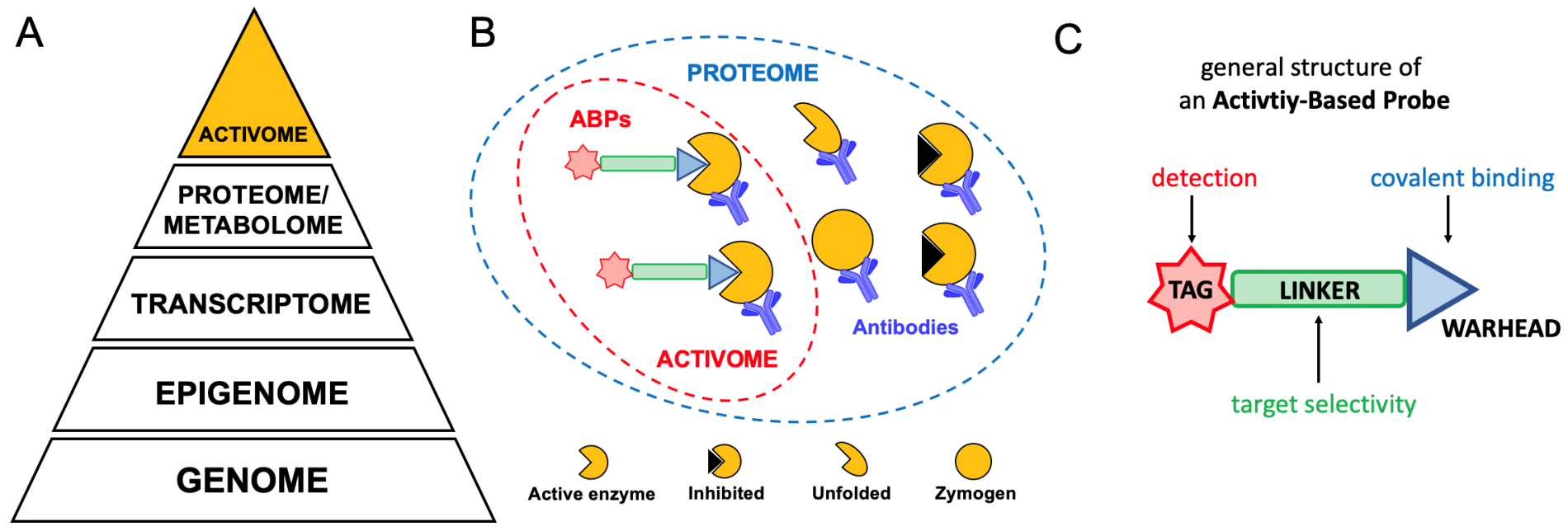
Enzymes are natural protein catalysts that accelerate the conversion of substrates into products. Depending on the type of catalyzed chemical reaction, enzymes are divided into several groups, among which we distinguish, for example, proteases - responsible for the hydrolysis (cleavage) of the peptide bond in peptide or protein substrates or kinases - responsible for transferring the phosphate group from one molecule to another, which very common is a protein. The diverse catalytic activity of enzymes means that they participate in virtually all biological processes. To maintain homeostasis from the level of a single cell to the interactions between individual organs, all enzymes must function properly.
Unfortunately, sometimes enzyme activity is dysregulated which leads to multiple pathological states e.g. cancer, diabetes, autoimmune diseases, or neurodegenerative diseases. To better understand the mechanisms underlying the pathogenesis of the disease and its course, it is necessary to understand the action of enzymes that are responsible for this disease. This matter gets complicated as enzyme activity is regulated on several levels, ranging from the number of their genes and transcript (DNA and RNA) to their abundance in the cell (proteome) to post-translational modifications (e.g. inhibition, degradation, etc.). Therefore, to fully understand the function of an enzyme, it is crucial to determine its catalytic activity. Methods for testing the activity of individual enzymes have been known for years (e.g. fluorescent activity-based probes, ABPs), but the problem is to test the activity of many enzymes simultaneously, in addition at the single cell level.
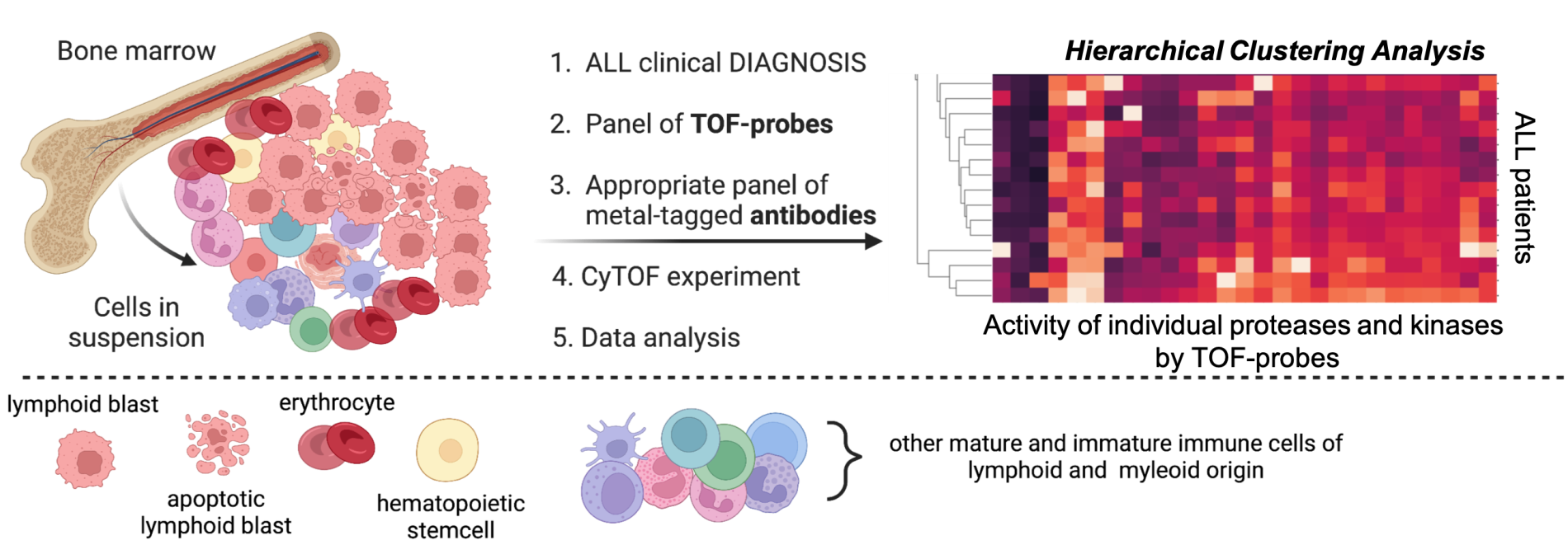
In our project, we plan to create a new chemical toolbox, a panel of activity-based probes tagged with a stable isotope of transition metals (from the lanthanide group). Such metal-tagged ABPs (we call them TOF-probes) will then be used in CyTOF technology, a modern analytical technique that allows the simultaneous analysis of more than 50 biomarkers (e.g. proteins/enzymes) at the resolution of a single cell. Currently, mass cytometry is used all over the world in immunology or oncology to study the concentration/presence of proteins in various biological samples. We plan to go a step further and use this technology to study the activity of individual enzymes at the level of individual cells (so- called: activome).
As a clinical model in our project, we will use bone marrow samples from patients with acute lymphoblastic leukemia (ALL). ALL is the most common type of childhood leukemia because as many as 75% of the youngest are diagnosed with this form of the disease. Moreover, ALL is the most common childhood cancer. Acute lymphoblastic leukemia is a cancer of the bone marrow, a system that deals with the production and maturation of blood cells from abundant erythrocytes and platelets to highly specialized lymphocytes and granulocytes. In ALL, healthy hematopoietic cells are displaced by mutated neoplastic cells, which in turn leads to impaired bone marrow function. Accurate diagnosis of this disease is based on the immune (presence of specific proteins on the surface of cells) and cytogenetic (presence of specific chromosomes and their mutations) profiling of bone marrow.
Currently, several types and over a dozen of molecular subtypes of ALL are characterized. Although the cure rate of children with acute lymphoblastic leukemia exceeds 90%, almost one in ten young patients does not respond to treatment. In our project, we want to dissect whether resistance to treatment is linked with aberrant enzymatic activity. For this purpose, we will analyze the activity of selected proteases and protein kinases, as these enzymes (in proteomic and genetic profiling) are most often associated with cancer. However, little is known about how their activity in leukemic cells, especially in childhood ALL. Moreover, the enzymatic analysis at the single-cell level will be correlated with the molecular subtype of ALL in individual patients. Therefore we will test whether these subtypes can be further differentiated based on enzyme activity. And if so, what are the clinical consequences of this in terms of diagnosis, treatment, and further prognosis of patients? We will conduct the research together with Prof. Wojciech Młynarski, head of the Department of Paediatrics, Oncology, and Hematology at the Medical University of Lodz. Prof. Młynarski is a world authority in the field of diagnosis and treatment of childhood ALL, but he is also a molecular biologist, so his contribution to this project will be invaluable. We believe that if this project is successful, our chemical technology for the simultaneous analysis of enzyme activity will also apply to other types of cancer, both hematological and solid tumors.