YOUR BROWSER IS OUT-OF-DATE.
We have detected that you are using an outdated browser. Our service may not work properly for you. We recommend upgrading or switching to another browser.
PI Marcin Poreba (in collaboration with prof. Boris Turk, Slovenia)
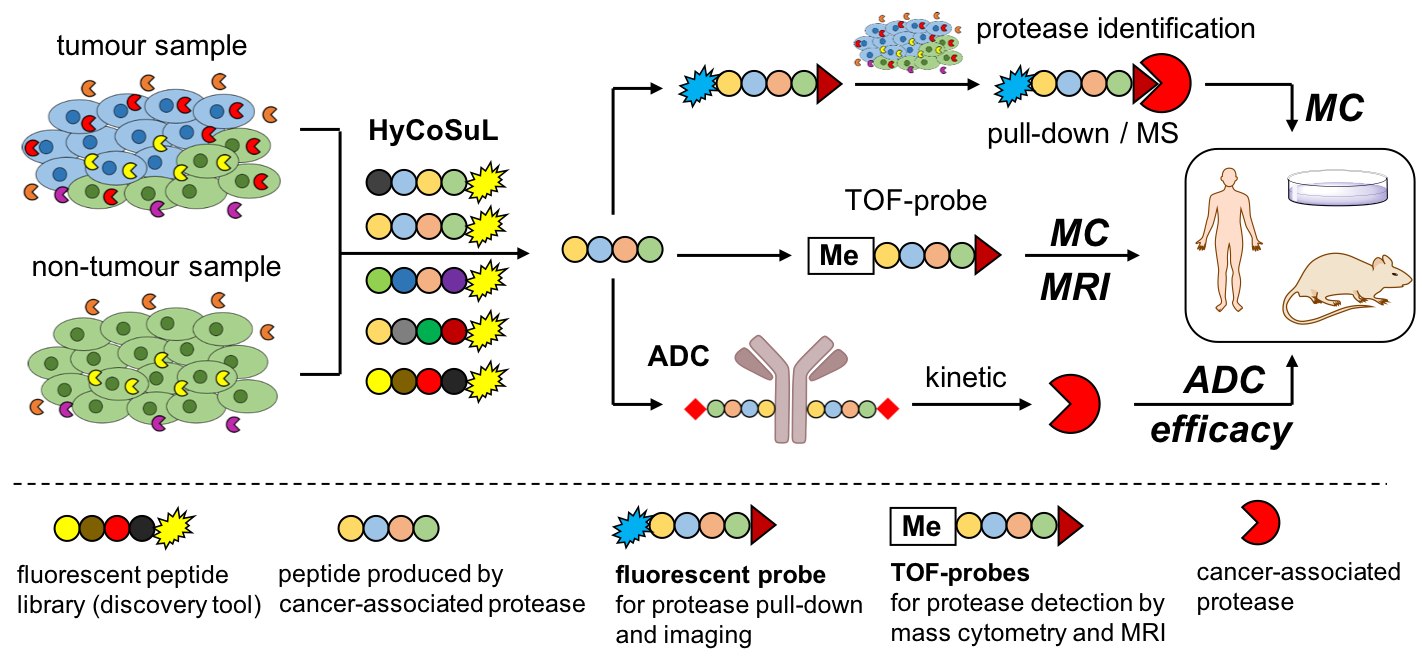
Nowadays breast cancer, and especially triple-negative breast cancer, is a very serious public health challenge. Moreover, the process of recovering from cancer depends on several factors, including early diagnosis, accurate cancer classification, and the choice of personalized treatment. To better deal with cancer, we need a more detailed insight into its biomarkers in order to understand their role in this disease. Most of such biomarkers have been discovered by analyzing the genome, proteome and metabolome of cancer cells, but this picture is not complete. We claim that the missing puzzle piece is ACTIVOME, which we define as a set of catalytically active proteins. Therefore our research project focuses on the active proteins (enzymes) with particular emphasis on proteolytic enzymes (proteases). The ultimate goal of this multidisciplinary project is to develop a cutting-edge strategy for the analysis of individual tumor cells in the context of the activity of individual proteases using mass cytometry. This knowledge will be used to obtain new generation of anticancer drugs, namely antibody-drug conjugates (ADCs). Therefore, our project is divided in three main work packages.
The first work package is the analysis of cancer cells by mass cytometry. Mass cytometry is a revolutionary technology that uses atomic mass spectrometry blended into flow cytometry format. The excellence of this method is that each metal isotope has its own peak on the mass spectrum, eliminating the problem of signal overlap and thus allowing more than 50 parameters to be monitored simultaneously at the single cell level. Most of the tools used in mass cytometry are antibodies linked to metal-containing polymers. However, for this project we will develop a set of metal-tagged chemical probes TOF-probes (Time-Of-Flight) which are compatible with mass cytometry and which were recently invented by our group. Unlike antibodies, our TOF markers will enable the detection of only active enzymes, i.e. those that can be used as specific molecular scissors to activate prodrugs. Moreover, the use of TOF-probes in combination with classical metal-labeled antibodies will provide a detailed picture of tumor cells landscape.
The resulting "tumor cell fingerprint" will be used in the second work package to develop antibody-drug conjugates that will be selectively activated by selected cancer-associated proteases. Such a system will enable precise drug delivery and activation only in tumor cells. Antibody-drug conjugates are currently one of the fastest-growing segments of oncology, but the vast majority of ADCs that have reached clinical trials have been highly toxic and have therefore not entered the market. In this project, we aim to develop a new generation of highly selective ADCs that will only be activated by cancer- associated proteases and therefore their systemic toxicity will be greatly reduced. The selectivity of these ADCs will be achieved through the use of highly selective peptide linkers that will bind the antibody to the drug. We plan to evaluate the therapeutic efficacy of these prodrugs in a panel of experiments ranging from in vitro studies in human and murine tumor cell lines to in vivo studies in live mice.
The third work package in our project will be the application of TOF-probes in MRI (Magnetic Resonance Imaging) analysis in order to accurately visualize neoplastic changes in a mouse model. Due to the fact that our TOF markers contain metals (mainly stable isotopes of transition metals, such as gadolinium), we decided to use them not only for mass cytometry, but also for tumor visualization, which will allow us to monitor the therapeutic effectiveness of our antibody-drug conjugates.
To achieve the project objectives, detailed research is required in several disciplines: chemistry, biochemistry, chemical biology, cancer biology, mass cytometry and magnetic resonance. That is why our research experience, scientific knowledge and infrastructure place our research groups in a unique position to successfully implement this highly innovative research project. Finally, our new concept of dissecting the cancer activome with TOF-probes goes beyond current technologies, therefore thereby creating a new research area with the potential to be a game changer in cancer research.