YOUR BROWSER IS OUT-OF-DATE.
We have detected that you are using an outdated browser. Our service may not work properly for you. We recommend upgrading or switching to another browser.
PI Małgorzata Firczuk (Mossakowski Medical Reseach Institute, PAS), PI at WUST Marcin Poreba
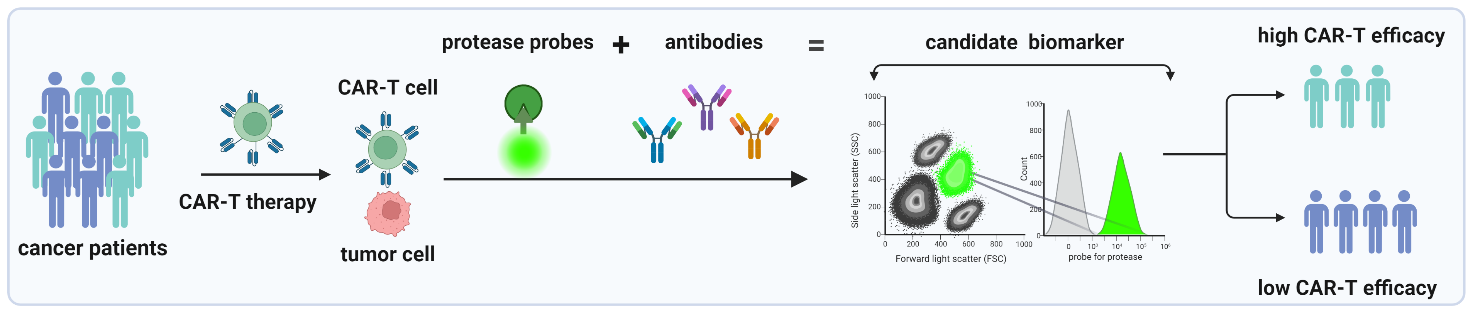
Reasons for Research Topic Selection. Cancer immunotherapy is an innovative cancer treatment strategy that employs the immune system, which possesses natural anti-tumor mechanisms. One type of immunotherapy is chimeric antigen receptor T-cell therapy (CAR-T therapy). CAR-T therapy is a highly personalized treatment approach that involves extracting the patient's own T-cells, modifying them in the laboratory by adding genes encoding CAR receptors, and reinfusing them into the patient's bloodstream. These modified CAR-T cells have the ability to kill cancer cells, making them a type of "living drug." Producing CAR-T cells individually for each patient is a time-consuming and expensive process. Currently, there are six registered CAR-T products, all used for treating hematological malignancies. CAR-T cell therapy was first introduced in 2017 and has already saved the lives of many patients who did not respond to other treatment methods. However, there are several challenges associated with CAR-T cell therapy. It is not effective for all patients and some experience life-threatening complications due to the therapy. Moreover, since it is a "living drug," the quantity of CAR-T cells does not always correlate with their activity, making it difficult to determine the appropriate dosage. Unfortunately, there is a lack of suitable biomarkers to assess the activity of the CAR-T cells produced and the CAR-T cells already present in the patient's bloodstream.
Project Objective and Research Hypothesis. The aim of this project is to identify new biomarkers for monitoring CAR-T cell therapy and evaluating the activity of autologous CAR-T infusion products. Based on preliminary results, we hypothesize that the activity of selected proteases can be used as biomarkers of CAR-T cell activity. Therefore, we aim to thoroughly investigate which proteases are activated in CAR-T cells upon their contact with cancer cells and synthesize probes enabling the evaluation of this activity.
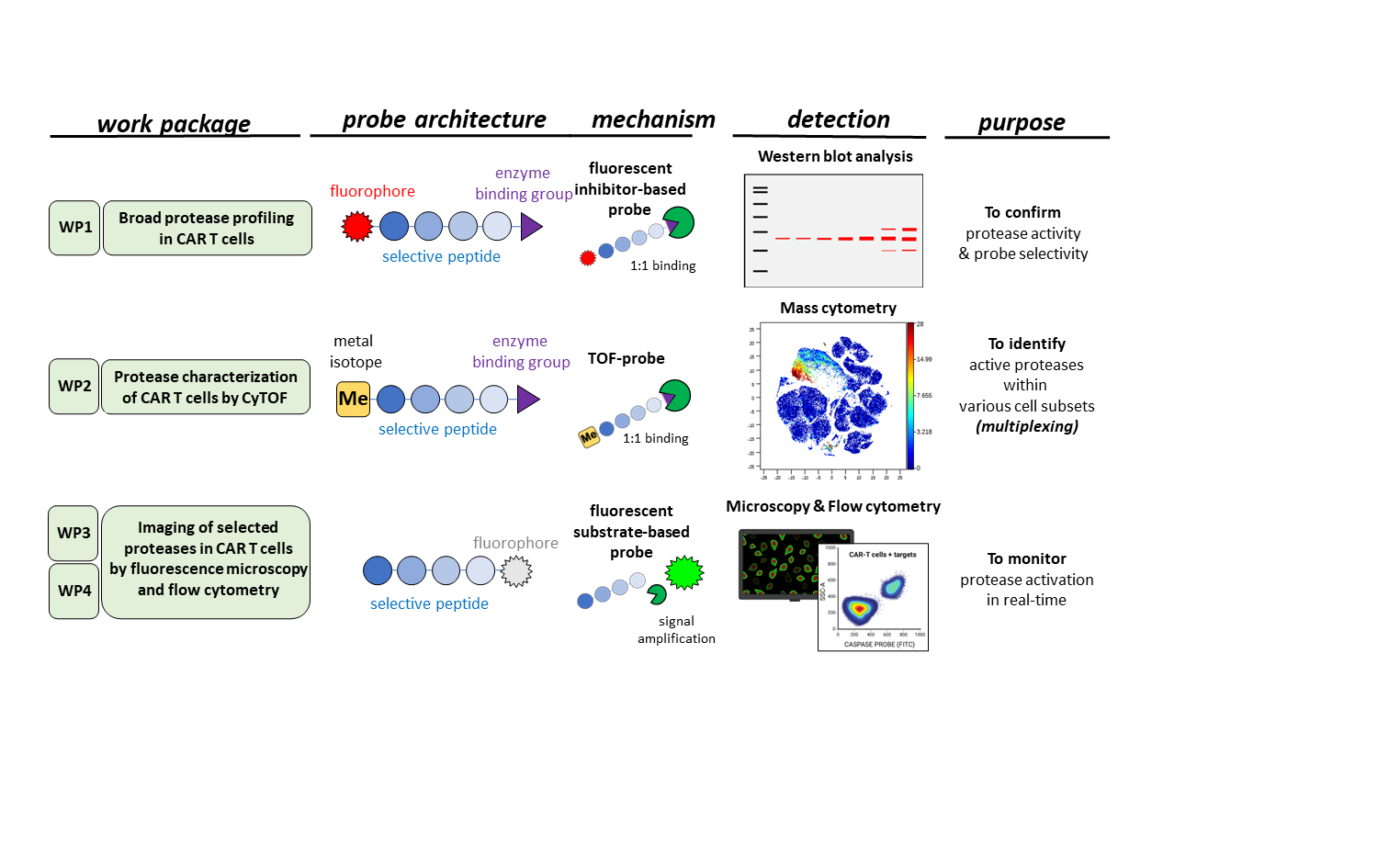
Research Description. The research will be conducted by two teams of researchers from different fields: experimental immunology and chemistry. In the initial stage of the project, in vitro studies will be conducted to comprehensively characterize the proteases activated in CAR-T cells following their contact with cancer cells. We will identify several proteases that could be used as biomarkers and synthesize probes for monitoring the activity of these proteases using mass and flow cytometry. In the subsequent stage of the project, utilizing these probes, we will precisely characterize the subpopulations of cells responsible for the most effective killing of cancer cells and identify their surface markers. Based on in vitro models, we will select markers of CAR-T cell activation in response to contact with cancer cells. In the final stage of the project, the selected markers will be evaluated in primary samples obtained from patients with hematological malignancies treated with CAR-T cell immunotherapy.
Key Expected Results. This study aims to enhance our understanding of the mechanisms underlying CAR-T cell activation, particularly the role of protease activation to utilize them as biomarkers of CAR-T cell activity. We firmly believe that achieving the goal of our project will contribute to the further improvement of innovative CAR-T cell-based immunotherapy.