YOUR BROWSER IS OUT-OF-DATE.
We have detected that you are using an outdated browser. Our service may not work properly for you. We recommend upgrading or switching to another browser.
PI Marcin Poreba
Alzheimer's disease (AD) is a devastating neurodegenerative disorder that affects millions of people worldwide, leading to progressive cognitive decline, dementia, and eventually death. Despite extensive research over the past few decades, effective treatments that can halt or reverse the course of the disease remain elusive. Current therapies, which primarily offer symptomatic relief, have limited efficacy in slowing the disease's progression, underscoring the urgent need for novel therapeutic strategies. In response to this challenge, we propose a research project with the goal of developing a new class of treatment for Alzheimer's disease: antibody-drug conjugates (ADCs). This innovative approach represents a significant departure from traditional therapies by combining the precision of antibody-based targeting with the potency of small- molecule drugs. The ADCs being developed in this project are specifically designed to target the pathological features of Alzheimer's disease with unprecedented accuracy. To evaluate their effectiveness and safety, these ADCs will be tested in mouse models of Alzheimer's disease, allowing us to study how they work in a living organism (in collaboration with prof. Urszula Wojda’s group from the Nencki Institute of Experimental Biology, Warsaw).
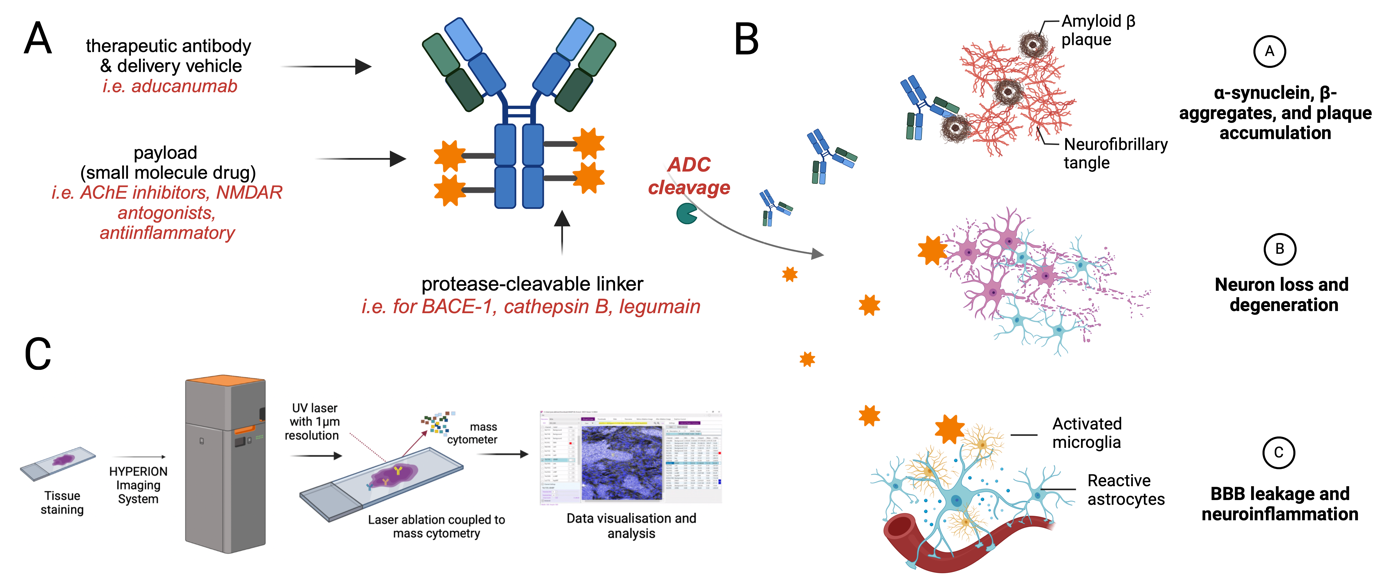
What are antibody-drug conjugates? ADCs are a type of targeted therapy that has already shown great promise in the field of oncology. They are composed of three main components: an antibody, a linker, and a drug. The therapeutic antibody is designed to specifically target proteins or cells associated with a disease, the drug is also the therapeutic agent intended to treat the disease, and the linker connects the two, controlling when and where the drug is released. In the context of Alzheimer's disease, the ADCs being developed will target proteins implicated in the disease's progression, such as amyloid-beta (Aβ) plaques and tau tangles. These pathological proteins accumulate in the brains of Alzheimer's patients, leading to neuroinflammation, synaptic dysfunction, and neuronal death. The innovative approach in our project involves combining a therapeutic antibody with a potent drug to enhance activity against Alzheimer's pathology. However, to improve delivery and minimize off-target toxicity, these components are conjugated using a protease-selective linker. This linker ensures that the drug is released specifically in the presence of Alzheimer's-associated proteases, allowing for precise drug activation only at the site of disease.
The role of proteases in Alzheimer's disease. A key feature of the proposed ADCs is their use of protease- sensitive linkers. Proteases are enzymes that break down proteins and play a crucial role in various biological processes. In Alzheimer's disease, certain proteases are upregulated and contribute to the formation of amyloid plaques and tau tangles. The research team aims to exploit these proteases as a trigger for the ADCs. By designing linkers that are selectively cleaved by Alzheimer's-associated proteases, the ADCs can be engineered to release their therapeutic payload only in the presence of these enzymes. This ensures that the drug is activated precisely where it is needed in the brain, reducing the risk of systemic side effects and increasing the efficacy of the treatment.
Advanced imaging techniques for mapping the disease. To optimize the targeting and effectiveness of these ADCs, the project will utilize a cutting-edge technology called imaging mass cytometry (IMC). IMC allows researchers to map the spatial distribution of proteins, cells, and other biomarkers within tissue samples at a high resolution. By applying IMC to brain tissue from Alzheimer's models, we can identify the exact locations where the proteases and pathological proteins are most active. This detailed mapping is critical for the development of ADCs, as it provides the information needed to design linkers that will only be cleaved in the regions of the brain affected by Alzheimer's disease. This level of precision in drug delivery is expected to enhance the treatment's effectiveness while minimizing potential harm to healthy brain tissue.
Potential impact of the research. The development of ADCs for Alzheimer's disease could revolutionize the treatment landscape for this debilitating condition. By combining targeted delivery, controlled drug release, and the ability to address multiple aspects of the disease simultaneously, ADCs offer a promising new approach that goes beyond the limitations of current therapies. If successful, this project could pave the way for more effective treatments not only for Alzheimer's disease but also for other neurodegenerative disorders that share similar pathological features. Moreover, the use of advanced imaging techniques like IMC could lead to new insights into the underlying mechanisms of these diseases, potentially opening up further avenues for research and therapeutic development.